With the long-term clinical trials still underway, and tens of thousands of deaths, along with millions of serious vaccine injuries (see US VAERS data and the EU equivalent), the FDA cynically and politically gave full approval this morning to the Pfizer-BioNTech mRNA experimental gene modification drug. This will pave the way for further imposition of medical tyranny and vaccine mandates.
The FDA claimed in 2020 that their ‘rigorous testing means final vaccine approval in 2024!’ LIE! The FDA’s Pfizer vaccine approval letter contains the dates of when studies were to be completed. NOT COMPLETED! Why did the timetable change? Why were dates suddenly no longer applicable and the vaccine approved by the FDA?1
The press reported that vaccine mandates are now legal for military, healthcare workers, college students and employees in many industries. New York City Mayor Bill de Blasio has now required the vaccine for all teachers and school staff. The Pentagon is proceeding with its mandate for all military service members.
But there are several bizarre aspects to the FDA approval that will prove confusing to those not familiar with the pervasiveness of the FDA’s regulatory capture, or the depths of the agency’s cynicism.
First, the FDA acknowledges that while Pfizer has “insufficient stocks” of the newly licensed Comirnaty vaccine available, there is “a significant amount” of the Pfizer-BioNTech COVID vaccine — produced under Emergency Use Authorization (EUA) — still available for use.
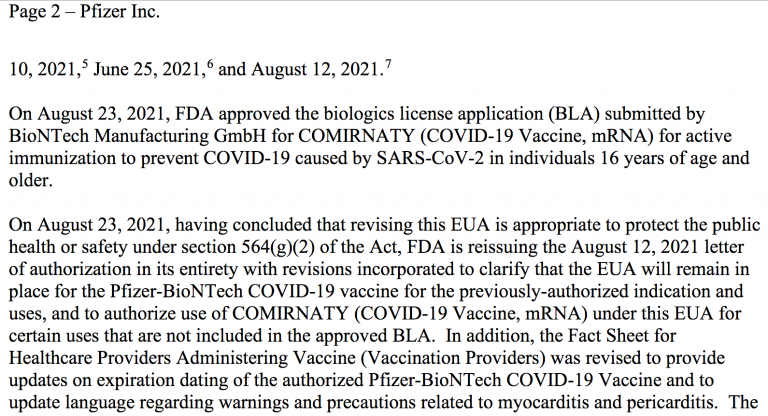
The FDA decrees that the Pfizer-BioNTech vaccine under the EUA should remain unlicensed but can be used “interchangeably” (page 2, footnote 8) with the newly licensed Comirnaty product.
Second, the FDA pointed out that the licensed Pfizer Comirnaty vaccine and the existing, EUA Pfizer vaccine are “legally distinct,” but proclaims that their differences do not “impact safety or effectiveness.”
There is a huge real-world difference between products approved under EUA compared with those the FDA has fully licensed.
READ: Wake Up! The FDA Has NOT Approved ANY COVID19 Vaccine
EUA products are experimental under U.S. law. Both the Nuremberg Code and federal regulations provide that no one can force a human being to participate in this experiment. Under 21 U.S. Code Sec.360bbb-3(e)(1)(A)(ii)(III), “authorization for medical products for use in emergencies,” it is unlawful to deny someone a job or an education because they refuse to be an experimental subject. Instead, potential recipients have an absolute right to refuse EUA vaccines.
U.S. laws, however, permit employers and schools to require students and workers to take licensed vaccines.
EUA-approved COVID vaccines have an extraordinary liability shield under the 2005 Public Readiness and Preparedness Act. Vaccine manufacturers, distributors, providers and government planners are immune from liability.
The only way an injured party can sue is if he or she can prove willful misconduct, and if the U.S. government has also brought an enforcement action against the party for willful misconduct. No such lawsuit has ever succeeded.
The government has created an extremely stingy compensation program, the Countermeasures Injury Compensation Program, to redress injuries from all EUA products. The program’s parsimonious administrators have compensated under 4% of petitioners to date — and not a single COVID vaccine injury — despite the fact that physicians, families and injured vaccine recipients have reported more than 600,000 COVID vaccine injuries.
At least for the moment, the Pfizer Comirnaty vaccine has no liability shield. Vials of the branded product, which say “Comirnaty” on the label, are subject to the same product liability laws as other U.S. products.
When the Centers for Disease Control and Prevention’s (CDC) Advisory Committee for Immunization Practices places a vaccine on the mandatory schedule, a childhood vaccine benefits from a generous retinue of liability protections.
But licensed adult vaccines, including the new Comirnaty, do not enjoy any liability shield. Just as with Ford’s exploding Pinto, or Monsanto’s herbicide Roundup, people injured by the Comirnaty vaccine could potentially sue for damages.
And because adults injured by the vaccine will be able to show that the manufacturer knew of the problems with the product, jury awards could be astronomical.
Pfizer is therefore unlikely to allow any American to take a Comirnaty vaccine until it can somehow arrange immunity for this product.
Given this background, the FDA’s acknowledgement in its approval letter that there are insufficient stocks of the licensed Comirnaty, but an abundant supply of the EUA Pfizer BioNTech jab, exposes the “approval” as a cynical scheme to encourage businesses and schools to impose illegal jab mandates.
The FDA’s clear motivation is to enable Pfizer to quickly unload inventories of a vaccine that science and the Vaccine Adverse Events Reporting System have exposed as unreasonably dangerous, and that the Delta variant has rendered obsolete.
Americans, told that the Pfizer COVID vaccine is now licensed, will understandably assume COVID vaccine mandates are lawful. But only EUA-authorized vaccines, for which no one has any real liability, will be available during the next few weeks when many school mandate deadlines occur.
The FDA appears to be purposefully tricking American citizens into giving up their right to refuse an experimental product.
While the media has trumpeted that the FDA has approved COVID vaccines, the FDA has not approved the Pfizer BioNTech vaccines, nor any COVID vaccines for the 12- to 15-year age group, nor any booster doses for anyone.
And the FDA has not licensed any Moderna vaccine, nor any vaccine from Johnson & Johnson — so the vast majority, if not all, of vaccines available in the U.S. remain unlicensed EUA products.
Here’s what you need to know when somebody orders you to get the vaccine: Ask to see the vial. If it says “Comirnaty,” it’s a licensed product.
If it says “Pfizer-BioNTech,” it’s an experimental product, and under 21 U.S. Code 360bbb, you have the right to refuse.
If it comes from Moderna or Johnson & Johnson (marketed as Janssen), you have the right to refuse.
The FDA is playing bait and switch with the American public — but we don’t have to play along. If it doesn’t say Comirnaty, you have not been offered an approved vaccine.
What this all means
The FDA approved Pfizer’s Biologics License Application (BLA). A BLA is the procedural process for “full approval” via the FDA. Pfizer may now directly market the mRNA injections to the public instead of relying on the government and media to push its agendas.
The mRNA injections can also now be sold in interstate commerce instead of governments buying up all the shots. The approved BLA will also increase Pfizer mRNA demand as it’s unlikely any new COVID-19 injections will receive EUA’s since one is now fully approved. Interestingly, the FDA granted Pfizer’s BLA but also extended its emergency use authorization (EUA) for “certain uses that are not included in the approved BLA.” That provision is specifically in reference to the EUA for children age 12 to 15.
This news changes only two things. First, it provides vaxx zealots celebration material and new talking points for defending their holy sacrament. It’s also a sure sign that those who refuse to be poisoned must be ready and willing to protect their lives and well-being by any means necessary.
This “full approval” was essentially purchased by the federal government. Pfizer profits will exceed $33.5 billion in 2021 from the mRNA injections alone, mostly courtesy of the U.S. and European governments. The facts about mRNA injections, the FDA and the Centers for Disease Control (CDC), however, remain unchanged. Keep this article handy to share with your Fauci-worshiping friends and family.
Unbelievably, the FDA also turned over the responsibility to conduct necessary vaccine trials on some of the most important subgroups, like pregnant women, to the manufacturer. Several mandatory studies that should have taken place before full approval still need to be conducted and it will be up to Pfizer to ensure their completion and report the results. In other words, Big Pharma now controls the outcome of key clinical research that will determine the effectiveness of their own product and has the ability to obscure results that could hold them liable or discourage people from taking their jab.
The FDA’s dereliction of duty didn’t end with that. In addition to all the other steps that were skipped, they never completed an advisory committee review because they claim there were no “concerns or controversial issues,” despite an overwhelming number of cases with adverse side effects like myocarditis.
Kim Witczak, a consumer representative on the FDA’s Psychopharmacologic Drugs Advisory Committee, questioned the decision to grant full authorization so quickly, and said that the meetings are important for public transparency and to scrutinize any data, in comments she made to The BMJ.
“These public meetings are imperative in building trust and confidence especially when the vaccines came to market at lightening speed under emergency use authorization.
The public deserves a transparent process, especially as the call for boosters and mandates are rapidly increasing. These meetings offer a platform where questions can be raised, problems attacked, and data scrutinized in advance of an approval.
It is already concerning that full approval is being based on 6 months’ worth of data despite the clinical trials designed for two years. There is no control group after Pfizer offered the product to placebo participants before the trials were completed.”
The president of the National Center for Health Research, Diana Zuckerman, also criticized the lack of transparency.
“It’s obvious that the FDA has no intention of hearing anyone else’s opinion. But if you make decisions behind closed doors it can feed into hesitancy. It’s important to have a public discussion about what kind of data are there and what the limitations are. As we think about risk versus benefit, we need to know.” – Zukerman said to to The BMJ.
The decision to fully approve the Pfizer vaccine is just the latest concerning decision by US Health Officials that have been either lying or outright wrong since the beginning of the pandemic. They have also been hiding relevant information from the public as it has been coming out the entire time.
The agenda behind this evil push remains a mystery. Money is obviously a priority, but is there a more diabolical intent?
CNN: Full approval of the Pfizer-BioNTech #COVID19 vaccine opens the door for more vaccination-only mandates throughout the U.S.pic.twitter.com/makGPUX9El
— Disclose.tv (@disclosetv) August 23, 2021
Joe Biden called on more companies to implement vaccine mandates for employees just hours after the Food and Drug Administration approved the COVID mRNA injection.
“As I mentioned before, I’ve imposed vaccination requirements that will reach millions of Americans,” Biden said Monday.
“Today, I’m calling on more countries – more companies, I should say – in the private sector to step up with vaccine requirements that’ll reach millions more people.”
“If you’re a business leader, a nonprofit leader, a state or local leader who has been waiting for full FDA approval to require vaccinations, I call on you now to do that. Require it,” Biden insisted.
“Do what I did last month. Require your employees to get vaccinated or face strict requirements,” he added, referring to his recent order for federal workers to get the injection.
Biden insisted that Americans will “be protected” if they get the jab even after admitting that some vaccinated people are still contracting COVID-19.
“There are cases where vaccinated do get COVID-19,” Biden admitted. “But they are far less common than unvaccinated people getting COVID-19. And most importantly, their conditions are far less severe.”
“With today’s FDA full approval, there’s another good reason to get vaccinated. So, please get vaccinated now,” he whispered, urging Americans to visit “vaccines.com” instead of the actual website vaccines.gov.
mRNA is gene therapy
Let’s be clear – the Pfizer and Moderna mRNA injections are the same product. The Moderna June 30, 2020 Quarterly Report filed with the U.S. Securities and Exchanges Commission tell you exactly what the mRNA injections are on page 70:
Currently, mRNA is considered a gene therapy product by the FDA. Unlike certain gene therapies that irreversibly alter cell DNA and could act as a source of side effects, mRNA-based medicines are designed to not irreversibly change cell DNA; however, side effects observed in gene therapy could negatively impact the perception of mRNA medicines despite the differences in mechanism. In addition, because no product in which mRNA is the primary active ingredient has been approved, the regulatory pathway for approval is uncertain.
Despite their mitigation attempts with the foregoing word salad, mRNA injections are in fact gene therapy.
On Aug. 27, the Orlando, Florida-based Liberty Counsel, perhaps the most respected Christian legal firm in the nation, issued a press release. Below is the release, published in full from Liberty Counsel.
WASHINGTON, D.C. – The Food and Drug Administration (FDA) has done a bait and switch by announcing it approved its “first COVID-19 vaccine” in order to push the “vaccine” mandates and protect the Pfizer pharmaceutical company from legal liability. However, there is currently no fully licensed COVID shot on the United States market.
Albeit confusing, and probably intentionally so, this summarizes the current status of the Pfizer-BioNTech shots:
- All existing Pfizer vials (in the hundreds of millions), remain under the federal Emergency Use Authorization (EUA) (meaning people have the “option to accept or refuse”);
- The third or “booster” Pfizer shot is identical to the above and remains under the EUA with limited use to certain categories of people;
- BioNTech received FDA approval for people ages 16 and above under the name Comirnaty, but there are no Comirnaty doses available in the United States;
- In other words, there is currently NO FDA approved COVID-19 injection available anywhere in the United States. Every COVID shot in America remains under the EUA law and thus people have the “option to accept or refuse” them; and
- Even when an FDA approved COVID shot becomes available, individuals are protected by federal law and many states laws from being forced to get these shots based on their sincere religious beliefs or conscience rights.
On August 23, the FDA issued two separate letters for two separate injections. There are now two legally distinct (Pfizer vs. BioNTech), but otherwise identical products.
The first letter is regarding FDA’s biologics license application approval for the Pfizer Inc/BioNTech COVID-19 injection which has been named Comirnaty. Yet Pfizer has not started manufacturing or labeling this drug for U.S. distribution, so it is not even available in the U.S.
It is unclear whether or not it is protected by a liability shield, but web-based U.S. government communication indicates that the same program that provides compensation for COVID vaccine-related injuries will apply Countermeasures Injury Compensation Program (CICP) rather than the National Vaccine Injury Compensation Program). At this point, there apparently has been no compensation paid to people injured by one of the COVID shots via the CICP.
The Pfizer injection, on the other hand, is still considered experimental under U.S. law. There is a legal difference between products approved under authorization of emergency use (EAU) compared with those the FDA has fully licensed. The FDA issued another letter for the existing Pfizer shots which confirms they are still under EUA, are not fully approved, and has a liability shield.
EUA-approved COVID shots have a liability shield under the 2005 Public Readiness and Preparedness Act. Vaccine manufacturers, distributors, providers and government planners are immune from liability. People who have been injured can file a lawsuit if they can prove willful misconduct, and if the U.S. government has also brought an enforcement action against the party for willful misconduct. No such lawsuit has ever succeeded.
That means people must be told the risks and benefits, and they have the right to decline a medication that is not fully licensed. The federal Emergency Use Authorization law and the FDA, including the FDA Fact Sheet, state unequivocally that each person has the “option to accept or refuse” the shots. In addition to federal law, the FDA includes the Nuremberg Code and the Helsinki Declaration on its website, emphasizing the fact that people cannot be forced to take experimental drugs without their full consent.
The FDA’s approval letter to Pfizer regarding the BioNTech injection, Comirnaty, states: “Under this license, you are authorized to manufacture the product, COVID-19 Vaccine, mRNA, which is indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older.”
This letter affirms the FDA has not approved the Pfizer/BioNTech injections for the 12- to 15-year age group, nor any booster doses for anyone.
Regarding the Comirnaty injection, the FDA admits, “We have determined that an analysis of spontaneous post marketing adverse events reported under section 505(k)(1) of the FDCA will not be sufficient to assess known serious risks of myocarditis and pericarditis and identify an unexpected serious risk of subclinical myocarditis.”
Therefore, follow up studies will be required with children six months to 15 years as well as six studies for up to five years regarding the adverse effects of myocarditis and pericarditis.
In addition, the FDA bypassed and disregarded the normal advisory committee and public comment process for this license.
The letter states, “We did not refer your application to the Vaccines and Related Biological Products Advisory Committee because our review of information submitted in your BLA, including the clinical study design and trial results, did not raise concerns or controversial issues that would have benefited from an advisory committee discussion” (emphasis added).
The FDA also acknowledges that while Pfizer-BioNTech has “insufficient supplies” (in other words, it is not currently available on the U.S. market) of the newly licensed Comirnaty vaccine actually available. However, the letter also states there is “a significant amount” of the Pfizer-BioNTech shots which has been produced under the EUA and will continue to be offered under the same EUA status.
In its approval letter, the FDA specifies the Pfizer shot under the EUA should remain unlicensed, is still available for use, and can be used “interchangeably” with the newly licensed Comirnaty product. According to the FDA, the newly licensed Comirnaty injection and the existing Pfizer shot, while “legally distinct,” are not any different in terms of their “safety or effectiveness.”
Despite whether these COVID shots are licensed or not, they cannot be mandatory under Title VII. In general, employee vaccine religious exemption requests must be accommodated, where a reasonable accommodation exists without undue hardship to the employer, pursuant to Title VII of the Civil Rights Act of 1964. Many people hold sincere religious beliefs against taking the COVID shots or taking those derived from or which used at any stage of the development aborted fetal cell lines.
Title VII defines the protected category of religion to include “all aspects of religious observance and practice, as well as belief.” 42 U.S.C. § 2000e(j). Moreover, as the EEOC has made clear, Title VII’s protections also extend nonreligious beliefs if related to morality, ultimate ideas about life, purpose, and death.
See EEOC, Questions and Answers: Religious Discrimination in the Workplace (June 7, 2008), (“Title VII’s protections also extend to those who are discriminated against or need accommodation because they profess no religious beliefs…Religious beliefs include theistic beliefs, i.e. those that include a belief in God as well as non-theistic ‘moral or ethical beliefs as to what is right and wrong which are sincerely held with the strength of traditional religious views.’
Although courts generally resolve doubts about particular beliefs in favor of finding that they are religious, beliefs are not protected merely because they are strongly held. Rather, religion typically concerns ‘ultimate ideas’ about ‘life, purpose, and death’”).
Liberty Counsel Founder and Chairman Mat Staver said, “The FDA has apparently tried to deceive people by issuing its two confusing letters without proper explanation. Despite the FDA’s slight of hand, there is currently no FDA approved COVID shot available in the United States. Even if there were an FDA approved COVID shot available, people still may request that employers, schools, and the military accommodate their sincerely held religious beliefs.”
Dr. David Martin warned the public that the Food and Drug Administration (FDA) has approved a coronavirus (COVID-19) vaccine that does not exist. “When members of the mainstream suggest that this approval has suddenly put what is sitting in freezers around the world into an approved status, that’s actually not true,” Martin said during his appearance on “Brighteon Conversations.” “There are still manufacturing guidelines that were not required for the EUA that would be required for a full-approved product…”
The FDA may have intentionally violated their express approval rules by granting approval of BioNTech’s COMIRNATY as they were required to have packaging and labeling approved at the same time. The key is in the label to have all information of the copied drug – vaccine except what needs to be changed such as manufacturer and location of manufacture.
The legal codes for references:
21 US CODE 355 (J) (2) (A) (ii) (II)
if the listed drug referred to in clause (i) has more than one active ingredient, information to show that the active ingredients of the new drug are the same as those of the listed drug.
Now how can they get away with this? It is in what else they left out.
21 US CODE 355 (J) (2) (V)
Identifying the Incrimination Evidence for a Prosecution
For a litigant/attorney to present a cogent case to a court of law for prosecuting an action against the FDA for the above the following evidence should be cited:
Information must be available to show that the labeling proposed for the new drug is the same as the labeling approved for the listed drug preferred to in clause (i) except for changes required because of differences approved under a petition filed under subparagraph (C) or because the new drug and the listed drug are produced or distributed by different manufacturers;
So the labeling would have to say EUA….. EMERGENCY USE AUTHORIZED” Emergency Use Authorized “ is on the Pfizer-BioNTech label.
Incriminating Evidence #1 When a drug – Vaccine is approved the company is allowed to advertise. See any BionNTech ads? NO
Incriminating Evidence #2 Location of manufacture is required to be disclosed. Please notice the location of manufacture is blanked out in the FDA letter.
Incriminating Evidence #3 Try to the Patient informational Pamphlet for COMIRNATY – YOU CANNOT GET ONE
Approved Drugs – Vaccines are required by law to have a Patient informational Pamphlet with. #1 All chemicals #2 All chemical structures #3 Mechanism of action #4 Drug interactions, #5 Location of Manufacture.
What is Missing?
If fully approved there would be words in the FDA press release of full acceptance as a new drug, such as: “The FDA reviewed your data and the FDA accepts your drug as safe” With such plain language words the drug becomes fully FDA accepted.
but THERE ARE NO WORDS LIKE THAT IN THE RULING
Such words also do not appear in the Summary Basis for Regulatory Action. As such, the August 23rd announcement by the FDA “approving” the Pfizer-BioNTech COVID-19 Vaccine, marketed as Comirnaty is not an approval recognised in law.
To confirm this, check the labeling now appearing on the Comirnaty packaging, which will still only show EUA….. EMERGENCY USE AUTHORIZED (photo right)
Will those diligent investigative journalists from the mainstream media be reporting on the above? Of course they won’t because the job of the mainstream media isn’t journalism! (PS)
Sources:
- https://www.thegatewaypundit.com/2021/08/cutting-corners-liberals-mindlessly-rejoice-fda-fully-approves-pfizer-vaccine-despite-poor-results-skipped-key-clinical-trials-advisory-committee-review/
- childrenshealthdefense.org
- https://creativedestructionmedia.com/news/2021/08/23/fda-cynically-grants-full-approval-for-pfizer-covid-vaccine-with-trials-years-away-from-completion/
- https://www.infowars.com/posts/biden-urges-companies-to-impose-vaccine-mandates-following-fda-approval/
See Also: